Global Life Sciences & Healthcare Festival - Day 2

Speakers
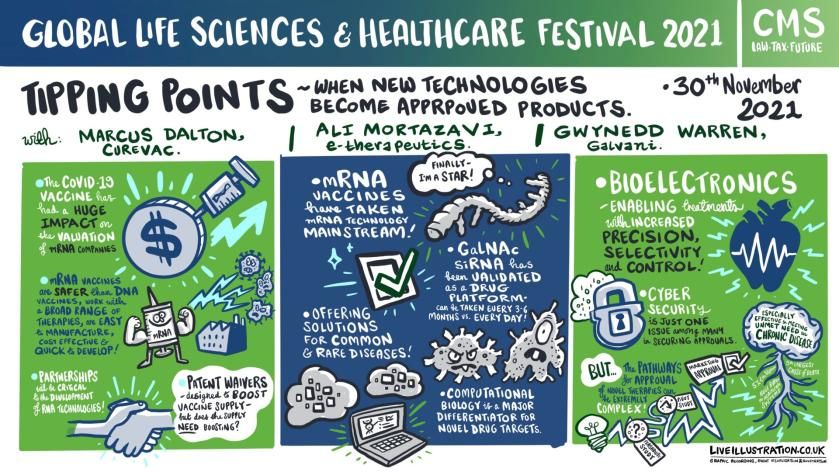
Tipping points – when new technologies become approved products
The pandemic has created a revolution that is disrupting healthcare and creating a wealth of opportunities for science and medicine and how it is delivered.
The whirlwind of activity caused by the outbreak of COVID-19 accelerated vaccine development and introduced unprecedented regulatory, political and legislative flexibility to get doses to the public.
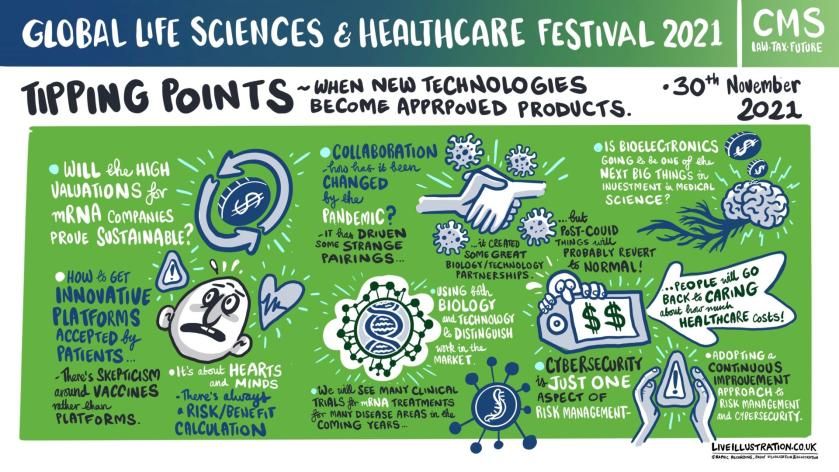
But the second day of Global Life Sciences & Healthcare Festival 2021 heard that the ‘astonishing’ pace of change leaves a slipstream swirling around IP, regulatory and legal concerns.
Marcus Dalton, vice president patents at leading global clinical-stage biopharmaceutical company CureVac, highlighted that the market capitalisation of RNA companies has boomed over the last 18 months – Moderna going from $6.3 billion to $100 billion – with increased M&A activity and big pharma looking to collaborate with RNA specialist companies.
Although there has been no patent conflict during the pandemic, he has identified a queue of IP cases relating to vaccines. He added: “I see IP litigation across the scope, but probably after the pandemic is over because of the political ramifications,” he added.
The Festival’s expert panel tackled issues such as cyber security, patent waivers and regulatory issues impacting tipping points in the developing platform technology that has emerged.
But, despite a range of concerns, life sciences continue to enjoy a buoyant outlook including a scientific surge from RNA techniques and capability that can be turned to treating chronic diseases and advanced to make gene editing products that repair or knock-out faulty genes.
The dynamic future of life sciences was underscored by Gwynedd Warren, of Galvani Bioelectronics, a medical research partnership between GSK and Verily Life Sciences, who outlined the potential from bioelectronic medicine, which focuses on signalling in the nervous system.
The sector has attracted healthy investment in technology and research but she advised that its fast-paced progress needed a collaborative approach with regulators as it was a novel field of medicine and science with a tough regulatory pathway.
“It is advisable to engage with your regulators because they'll be unfamiliar with the approach and with the system that you're intending to bring to market. You need to introduce them to the benefits as well as the challenges early,” added Warren, who is Galvani’s vice president head of legal and IP.
Jens Wagner, Global Co-Head CMS Life Sciences & Healthcare Sector, said: “We had some fantastic presentations and panel discussions that show what a dynamic era life science is in.
“The scale of collaborations and new developments are impressive and it was fascinating to learn about novel areas of research and consider the implications they have on the future of life sciences.”
Festival highlights