Global Life Sciences & Healthcare Festival - Day 1

Speakers
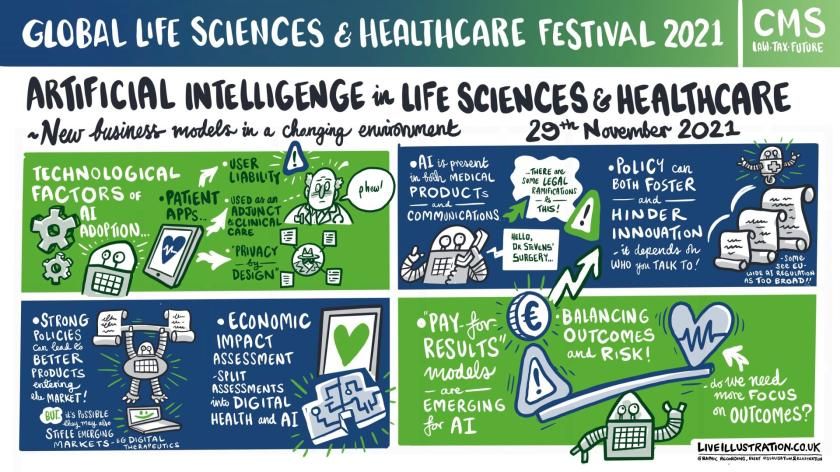
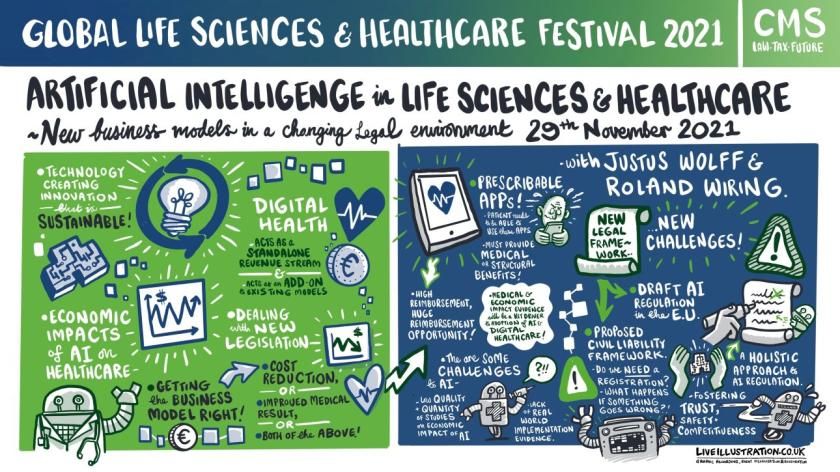
Artificial Intelligence in Life Sciences and Healthcare: New business models in a changing legal environment
The healthcare technology goldrush promises a brighter healthcare future but complex legal, economic, political and security issues still rage across its landscape.
The challenges of developing Artificial Intelligence (AI) across healthcare were put under the microscope on the opening day of the Global Life Sciences & Healthcare Festival 2021.
AI capabilities in pharma and medtech are racing ahead with legislation, regulatory and business modelling playing catch-up as the sector expands.
A high-level panel from industry, technology and legal wrestled with the dynamics of a sector that is seen as a potential saviour for the crippling stresses on global healthcare systems and its ability to become a significant revenue generator.
Digital health applications have been developed to cover every aspect of health and Justus Wolff, manager digital health of the Hamburg-based Syte Institute, the digital health strategy specialists, told the audience that the sector offered huge revenue opportunities.
But he cautioned that despite new players entering the market, there was still limited economic evidence of the impact of some digital apps and more research was needed.
It is easy to see the benefits of speed, convenience and connectivity but issues around civil and product liability still need detailed evaluation, said Dr Roland Wiring, CMS partner, who examined the draft EU legislation into AI.
He warned that the law, which is expected to be enacted in 2023, could place barriers in the path of innovation and even introduced a double certification process that could slow down progress.
“We have a dynamic sector and there are certain risks and liability to the new regulations on market access,” he said. “But there are also opportunities. Regulatory changes are coming and it is important for companies to monitor them and adopt them early to have success.”
Martin Gouldstone, chief business officer of Sensyne Health, said that his company’s apps, which deploy anonymised NHS England patient data to guide and support GPs, were CE approved and were not considered high risk. But, although companies in the UK are not governed by EU regulations, they need to be mindful of the legislation.
Dirk Spacek, a CMS partner and new business models expert, added that the rapid uptake of technology during the pandemic had left ‘grey areas’ of interpretation about medical device regulation and the influence of machine learning on a product’s status.
The medical and economic boost from digital health implementation was proved during the pandemic but its full adoption needs to come with an assessment of its real world impact, its ability to drive significant change while ensuring it has ‘privacy by design’.
Nick Beckett, Global Co-Head CMS Life Sciences & Healthcare Sector, added: “Technology offers the life sciences sector amazing opportunities to improve care but we need to give it every chance of succeeding by having the right legal and business framework.
“This is an emerging sector and the debate was fascinating and showcased the determination of the sector to get the best from a golden opportunity.”
Festival highlights






_840x420.jpg)